Nitrosamine Analysis
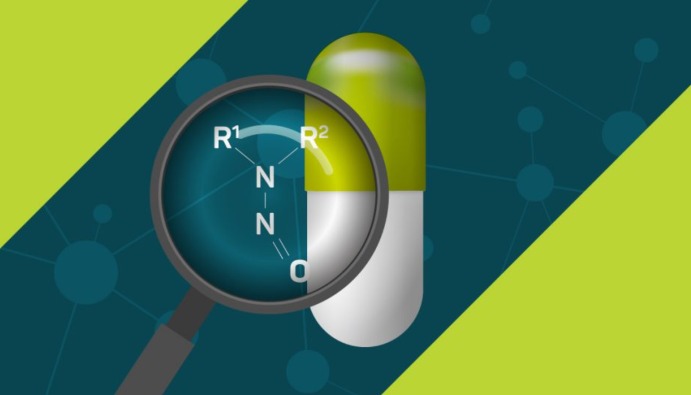
They are organic compounds with the general formula R2NNO or RNHNO. They are a group of organic chemicals formed by the interaction of nitrites with amines or amides in the body and are potentially carcinogenic. Nitrosamine and N-nitrozo compounds cause cancer in the liver and kidneys.
In the pharmaceutical industry, nitrosamines can be formed during the production and chemical reactions of active and excipients. Products containing NDMA, NDEA, NMBA, NDEA and other components have been detected in over 30 products used in the treatment of hypertension, type II diabetes and heart attacks. In June 2018, international authorities (FDA and EMA) initiated controls after nitrosamines were detected in the angiotensin II receptor blocker valsartan and some of the pharmaceutical products containing this active substance.
In September 2019, given the presence of N-nitrosamines in sartan with tetrazole rings, as well as in other active substances/medicinal products, the EMA initiated a review on the detection, management and prevention of the presence of N-nitrosamines in medicinal products for human use in accordance with Article 5(3) of Regulation (EC) No 726/2004.
FDA and EMA have issued guidelines to determine acceptable levels of these nitrosamine impurities and have initiated a work with the industry to check products that are on the market or will be on the market.
In this context, the Turkish Medicines and Medical Devices Agency (TİTCK), together with international health authorities, is requesting assessments from companies for all medicinal products on the market.
With our team of experts, methods were validated for 18 different nitrosamine analyses in LC-MS/MS and 6 different nitrosamine analyses in GC-MS/MS simultaneously in active/auxiliary substances, packaging materials and finished pharmaceutical products in GLP standards.