
BLOG
KATEGORİDEKİ DİĞER YAZILAR
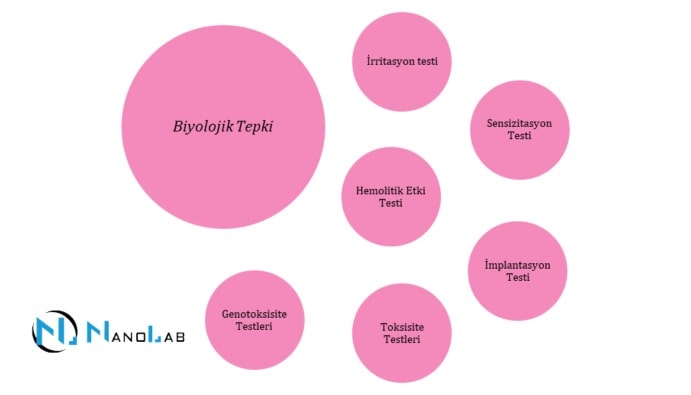
Medical devices are products that interact directly or indirectly with the human body. Therefore, biocompatibility tests play a critical role in ensuring the biological safety of medical devices, protecting the health of users and ensuring the effectiveness of the product. Biocompatibility means the ability of a material or device to function safely after contact with the human body without adversely affecting health.
Biocompatibility testing for medical devices is the assessment of the effects of the interaction between the devices and the tissues and tissues of the patient being treated as part of the overall safety assessment of the devices. Analytical chemistry, in-vitro testing and animal models are used for this testing. There are several factors that influence biocompatibility, such as the chemical and physical nature of the component materials, the types of patient tissue that will be exposed to the devices and the duration of this exposure.
Individual materials used to make medical devices may be partially biocompatible, but the combination of various materials may cause a toxic reaction. Therefore, the medical device must be assessed for biocompatibility.
Testing the biocompatibility of medical devices is vital to ensure that devices can be used without harming human health. These tests assess the safety and efficacy of devices, identifying the following critical risks in advance:
The ISO 10993 series are the main standards used to assess the biocompatibility of medical devices.
Nanolab Laboratories Group continues to provide services within the scope of Biocompatibility Tests in Medical Devices. We also provide services in In Vitro Cytotoxicity Tests.
Contact us for more information.
You can follow us on LinkedIn for up-to-date news and posts about our services.
Follow our Instagram account to be informed about our latest blog posts.