
BLOG
KATEGORİDEKİ DİĞER YAZILAR
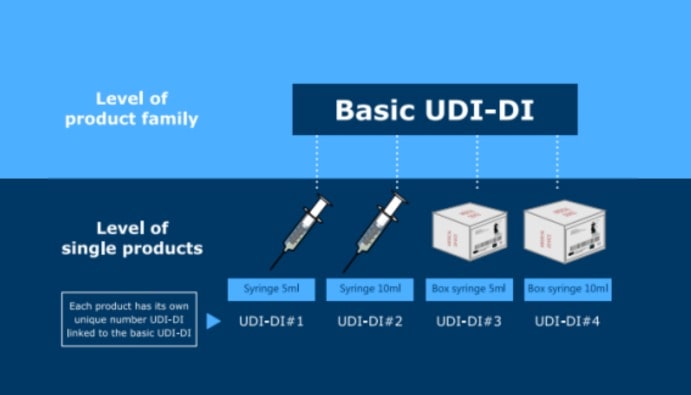
The Unique Device Identifier (UDI) is a unique identifying number for each medical device and is a system developed to ensure the traceability of medical devices, increase safety and improve efficiency in the healthcare system. In the European Union, the use of UDI is mandatory under the EU-MDR (Medical Device Regulation) and EU-IVDR (In Vitro Diagnostic Device Regulation). The UDI system facilitates the tracking of devices throughout their lifecycle and allows for the accurate collection of all product-related information.
UDI is a code or number used to identify a medical device or in vitro diagnostic device, uniquely marking the device. The UDI usually consists of two parts:
A UDI is usually located on the device in the form of a barcode or RFID (Radio Frequency Identification) tag. This allows healthcare providers and users to quickly identify the device.
The UDI system improves safety and quality control processes for medical devices by enabling more efficient and safer monitoring of medical devices. The main benefits of this system are as follows:
The UDI system allows to quickly identify problems that may arise during the use of devices. The correct implementation of UDI makes it easier for healthcare providers to review the history of devices and identify the source of possible malfunctions or errors. This increases the safety of patients.
Healthcare providers can use the UDI system for the proper management and storage of medical devices. The location of devices in inventory and their utilization can be tracked, enabling efficient management of resources. This can provide information on the expiry date and duration of use of the devices and facilitate stock management.
UDI improves device data collection and reporting. With UDI, healthcare professionals can access accurate and transparent information about devices. In addition, the use of UDI enables better health data analysis.
European Union regulations such as EU-MDR and EU-IVDR have made it mandatory for medical devices to use UDI. This makes it easier for manufacturers to comply with regulations. UDI helps manufacturers and distributors fulfill legal requirements and quickly provides the information needed for audits.
In the European Union, the EU-MDR and EU-IVDR regulations mandate the use of UDI. These regulations aim to improve the traceability and safety of medical devices. Manufacturers must create a UDI number for each device and place this number on product labeling before placing their devices on the market.
The EU-MDR mandates UDI to improve the safety of medical devices and reduce the impact of adverse events. When medical devices are classified according to specific classes (I, IIa, IIb, III), UDI requirements will also be subject to specific rules. For high-risk devices, the UDI should have more detailed information.
EU-IVDR has similarly mandated the use of UDI for in vitro diagnostic devices. The UDI requirement is also applied to ensure the traceability and safety of in vitro diagnostic devices.
EUDAMED (European Database on Medical Devices) is the central database for the traceability of medical devices and in vitro diagnostic devices in the European Union. UDIs are registered in the EUDAMED database, where they are traceable throughout the lifecycle of the devices. EUDAMED collects UDIs in a centralized location and facilitates processes such as device recalls and safety reporting.
Nanolab Laboratories Group continues to provide services within the scope of Medical Device Analysis.
Contact us for more information.
You can follow us on LinkedIn for up-to-date news and posts about our services.
Follow our Instagram account to be informed about our latest blog posts.